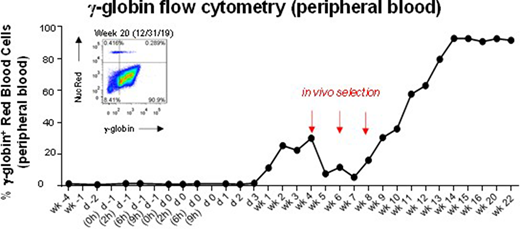
Current gene therapy or genome editing studies for hemoglobinopathies require highly sophisticated medical facilities to perform hematopoietic stem cell collections / selections and genetic modifications. In addition, patients receive high-dose chemotherapy to facilitate engraftment of gene-modified cells. Thus, current gene therapy protocols will not be accessible to most patients suffering from hemoglobinopathies. Here we describe a highly portable and scalable approach using in vivo hematopoietic stem cell (HSC) gene therapy to potentially overcome these limitations. The central idea of our in vivo HSC gene therapy approach is to mobilize HSCs from the bone marrow, and while they circulate at high numbers in the periphery, transduce them with an intravenously injected HSC-tropic, helper-dependent adenovirus HDAd5/35++ gene transfer vector system. Transduced cells return to the bone marrow where they persist long-term. Transgene integration is either achieved by a Sleeping Beauty transposase (SB100x) in a random pattern or by homology-directed-repair into a safe genomic harbor site. Currently, an in vivo selection system (involving the mgmtP140K gene/low-dose O6BG/BCNU) is employed to achieve 80-100% marking levels in peripheral blood cells. We demonstrated safety and efficacy of our approach in mouse models for thalassemia intermedia, Sickle Cell Disease, and hemophilia A, where we achieved a phenotypic correction. We now present data in 3 rhesus macaques. We show that treatment with G-CSF/AMD3100 resulted in efficient HSC mobilization into the blood circulation and subsequent intravenous injection of the HDAd5/35++ vector system (total 1-3 x1012 vp/kg, in two doses) was well tolerated. The longest follow up thus far is 24 weeks after in vivo HSC transduction with a human-gamma-globin expressing HDAd5/35++ vector. After in vivo selection with O6BG plus low dose (10 to 20 mg/m2) of BCNU, a dose that is up to 100-fold lower than what is used for autologous transplantation protocols, gamma-globin marking in peripheral red blood cells rose to ~90% and was stable for the duration of the study (see Figure). gamma-globin levels in red blood cells were ~18% of adult alpha1-globin (by HPLC). No abnormalities in genome and transcriptome analyses of animal #1 were found at the time of scheduled necropsy. We show that a new prophylaxis regimen (dexamethasone, IL-6R, IL-1bR antagonists, saline bolus IV) was able to mitigate all side effects associated with intravenous HDAd5/35++ vector administration. Analysis of day 3 bone marrow showed 30% transduced HSCs. Vector DNA biodistribution studies demonstrated very low or absent transduction of most tissues (including testes and CNS). Analysis of bone marrow showed efficient, preferential HSC transduction and re-homing of transduced CD34+/CD90+ cells to the bone marrow. At week 4, about 5% of progenitor colony-forming cells demonstrated stable transduction with integrated vector, and this frequency increased after starting the in vivo selection. The level of human mgmtP140K mRNA expression in PBMCs also increased after in vivo selection.
In summary: Using a new and optimized prophylaxis regimen intravenous delivery of HDAd5/35++ was very well tolerated without any cytokine activation. We saw efficient transduction of HSCs and efficient in vivo selection of transduced progenitors with low dose O6BG/BCNU. This is the first proof-of-concept study that in vivo HSC gene therapy could be feasible in humans without the need of high-dose chemotherapy conditioning and without the need for highly specialized medical facilities. This approach would provide a major advance for the gene therapy and genome editing field and allow the necessary portability and accessibility to reach patients in places with limited medical resources.
Radtke:Forty Seven INC: Consultancy. Kiem:Umoja: Membership on an entity's Board of Directors or advisory committees; Rocket Pharma: Membership on an entity's Board of Directors or advisory committees; Vor Biopharma: Membership on an entity's Board of Directors or advisory committees; Enochian: Membership on an entity's Board of Directors or advisory committees; CSL: Consultancy; Magenta Therapeutics: Consultancy; Homology Medicines: Membership on an entity's Board of Directors or advisory committees. Lieber:Ensoma, Inc: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.